Investigations into the death of a woman at Buderim Private Hospital are underway.
A contaminated saline product is being investigated as a possible cause of the death of an elderly woman at Buderim Private Hospital on Queensland’s Sunshine Coast.
According to the ABC, Queensland Health has told hospitals and clinics across the state to stop using two InterPharma sodium chloride products immediately and to remove them from all clinical areas.
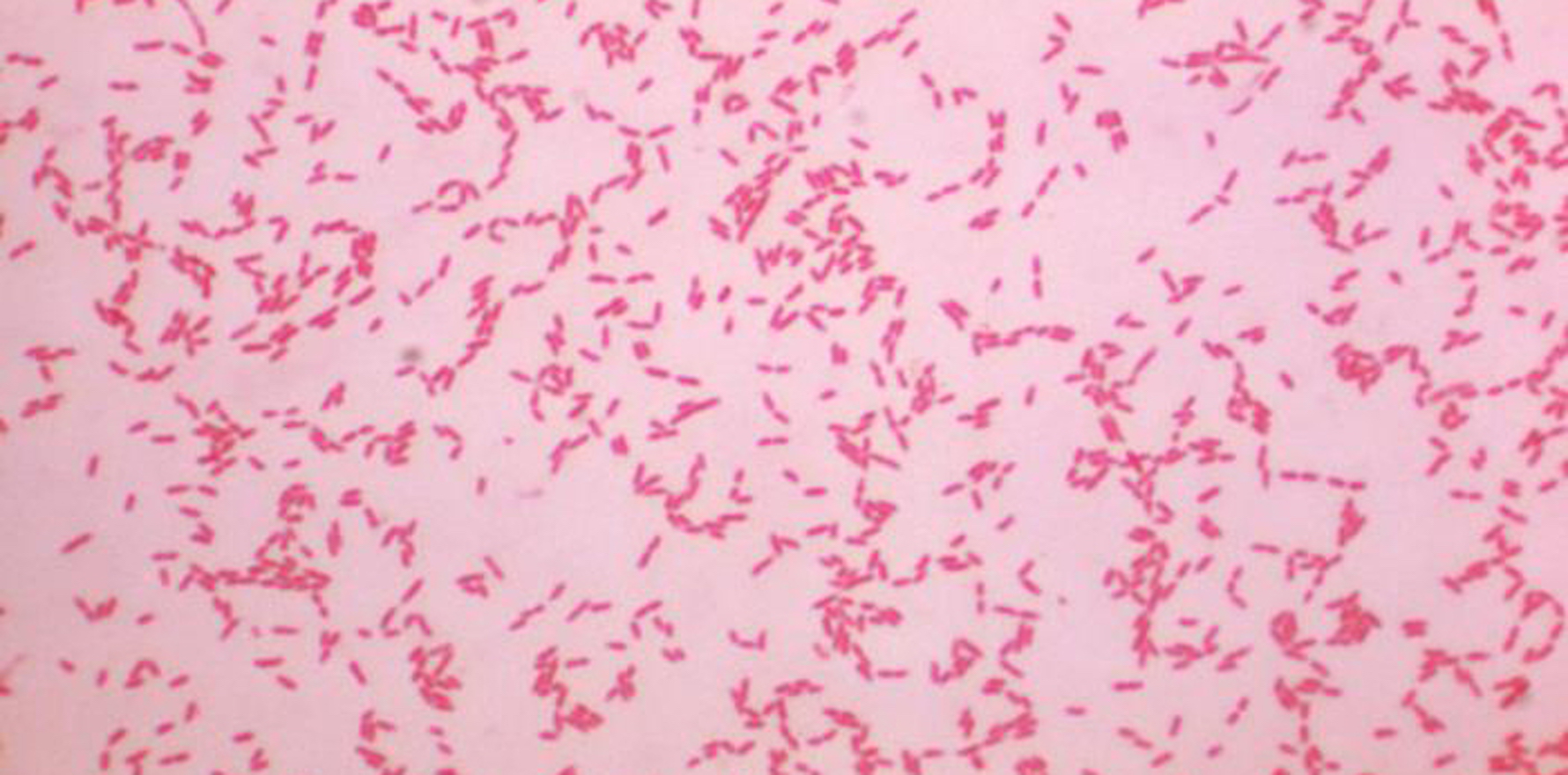
The imported products are believed to be linked to a hospital outbreak of a bacteria, Ralstonia pickettii, nationwide. There are at least 44 infections believed to be linked to the contaminated saline, with one confirmed Ralstonia case and five probable cases identified in Queensland.
“We are investigating all recent Ralstonia infections in Queensland to determine if any are linked to the national outbreak,” said Queensland Health in a statement. “We are participating in outbreak investigation meetings being led by NSW Health.”
In Queensland parliament this morning, Health Minister Shannon Fentiman said the bacterium outbreak was initially identified in New South Wales in September.
“It is especially concerning to those with significant underlying medical conditions or who have implanted medical devices,” she said.
“This is a national issue … and involves products imported from overseas. I can confirm that these products have been imported from India and Greece.
“In mid-September, NSW health authorities asked Australian states to be on the lookout for Ralstonia cases after a cluster of infections was identified, but with no apparent cause.
“The organism was identified in the blood of an elderly patient in a Queensland private hospital, who has subsequently passed away.”
Ms Fentiman told parliament she was advised the particular brand of saline had been in use in seven Queensland Health hospitals and facilities.
The TGA issued an urgent quarantine notice for the two products – InterPharma’s sodium chloride 0.9% 30ml amp and the 0.9% 10ml amp – saying it had “been made aware of possible contamination”.
“This is due to reported cases of Ralstonia pickettii in multiple jurisdictions. These products should be removed from use immediately and placed in quarantine until further notice.”
Do you have a story tip for us, or a topic you would like to see us cover? Contact the editor at editor@healthservicesdaily.com.au.